Product specification or Customer Requirements
Product specifications or customer requirements are used to formalise all technical, quantitative and regulatory requirements applicable to finished products. They establish a compliance framework to ensure that the final product meets customer requirements, applicable legal obligations and the company's internal quality standards.
These specifications may include, for example:
- quantitative constraints on certain ingredients, such as a salt content of less than 2%;
- restrictions on origin or provenance, such as a ban on the use of eggs from Germany;
- specific labelling rules that apply to certain products;
- bans on additives or substances, such as titanium dioxide (E171), which is not authorized in France.
Thus, the customer requirements plays a central role in ensuring product compliance throughout the production chain.
Creating a product specification
As with other entities, you can create a specification from any product space by clicking on ‘+ Create...’ and then selecting ‘Product specification’:
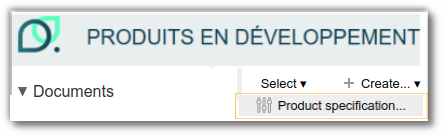
Once your ‘Product Specification’ entity has been created, several lists are available to you, functioning in the same way as those for other entities.
Properties: name your specifications, enter the associated regulatory code, add a description, define a compatibility filter, link your ‘Product Specification’ to another (for example, a product requirement specific to a country of distribution or a particular activity).
Documents: Import your documents (files, reports, supporting documents) and link them directly to your specification.
Allergens: Add forbidden allergens in voluntary or involuntary.
Nutrients: Define the specific nutrients to be taken into account and their maximum or minimum dose.
Physicochemical: Enter the physicochemical characteristics to be taken into account and their maximum or minimum dose.
Claims: Add authorized, forbidden or controlled claims.
Forbidden ingredients: Create alerts to flag the use of forbidden or tolerance-subject ingredients.
CLP: Create restrictions on hazard statements (e.g. H319) in accordance with CLP regulations.
Score: Create score criteria that will be formulated on associated entities.
Labeling rules: Define specific labelling rules that will only apply to products related to the specification.
Note : Specifications are automatically reformulated every day at 11 p.m. You can also manually reformulate the product at any time. If a list does not appear in the specification template, it can be added to the template available in [Administration].(/administration/administration.md).
Associate a product with a specification
To link a product specification to an entity, open the finished product and edit its properties. In the ‘Specifications’ field, select the desired specifications.
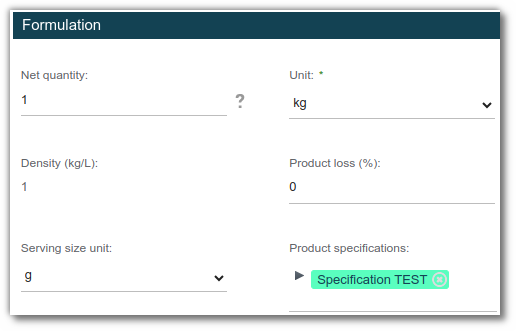
Complete a product specification
Requirement levels and alert messages
On each list, you can indicate a requirement level :
Forbidden: alert regarding the prohibition of using a characteristic in a recipe. In the case of dosage, you can:
Leave the maximum dosage field blank if the characteristic is completely forbidden (0%).
or enter a value if a maximum usage threshold is authorized.
Tolerated: alert on the tolerance of a characteristic in a recipe. As with the concept of ‘Forbidden’, in the case of dosage, you can:
Leave the maximum dosage field blank if the characteristic is completely forbidden (0%).
or enter a value if a maximum usage threshold is permitted.
Authorized: defines a list of positive characteristics. Characteristics not included in the specification will automatically be considered forbidden in the recipe. Therefore, all ingredients included in this specification must have the requirement level ‘Authorized’. Example: in certain geographical areas (e.g. Africa), only specific categories of ingredients may be used.
Information: This option simply allows descriptive information to be associated with an ingredient, without affecting its authorization or dosage.
If the product associated with the specification contains a characteristic whose quantity significantly exceeds the authorized limit defined in the specification, an alert is displayed in the list in question and on the characteristic line.
A notification is also generated in the completion wheel.
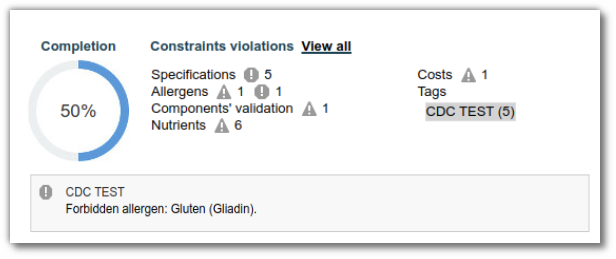
In the “Messages” section, you can enter the text that will be displayed when a compliance alert is triggered.
Allergens
The list of allergens allows you to prohibit the presence of an allergen, whether direct (contains) or indirect (may contain). Select ‘No’ if you wish to prohibit an allergen.
- Quantity: allows you to define a threshold above which the allergen is considered to be present in the entity (e.g. rinsed and non-rinsed products in cosmetics). When the quantity exceeds this threshold, the ‘contains’ column for the entity displays 'yes', otherwise ‘no’. This feature is used in cosmetics when allergens are directly linked to ingredients in the administration, in order to manage allergen labelling thresholds.
Example: do not label linalool below 100 ppm for rinsed products.
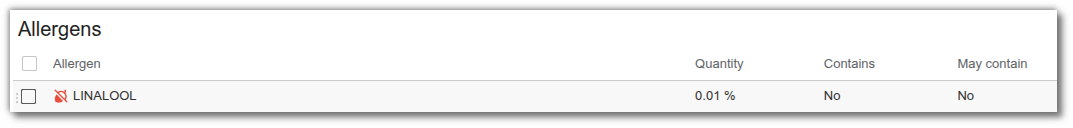
Contain: select ‘yes’ if you only want to manage labelling thresholds related to quantity, or select ‘no’ to generate compliance alerts.
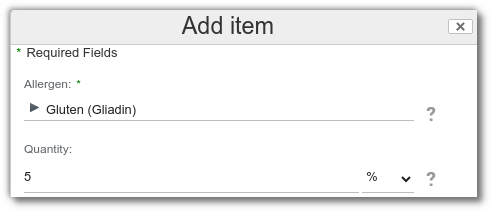
May Contain: select ‘no’ if you want to prohibit an allergen in incidental presence.
Example: prohibit gluten when its content exceeds 5%.
Note: If you have specified a regulatory threshold on the administration's allergen list and you override it with a threshold in the specification, the specification threshold will be used. To specify a regulatory threshold on the allergen list, click here.
Nutrients
From the list of nutrients, select a nutrient to ban, then:
- Threshold value: in the Mini or Maxi section
Target value: type of value to which the threshold should be applied.
1 serving
% RI per portion
Prepared value (per 100 g)
Prepared value per serving
Labeled serving
Labeled prepared value (per 100 g)
%RI per labeled serving
Labeled value (per 100 g)
Labeled prepared value per serving
Example: forbidden sodium when its content exceeds 25 mg per serving.
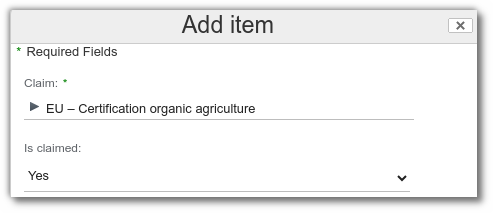
Claims
From claims list, add the desired claim and its status, then save the change. Example: Organic agriculture
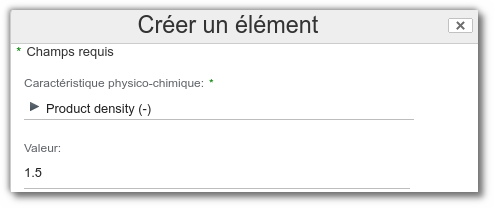
Physicochemical
From the Physicochemical list, select the physicochemical property to be prohibited, enter its threshold value (with Min and Max), then save your changes.
Example: Alert if density is greater than 1.5
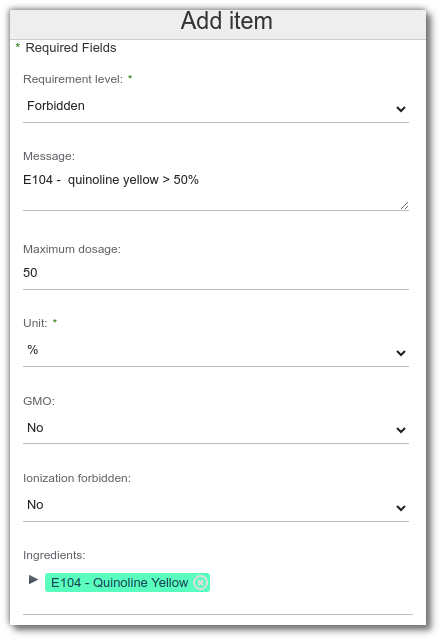
Forbidden ingredients
From forbidden ingredients list, select:
- Ingredients: Several ingredients can be selected to take into account the total amount.
- Dosage: Maximum and/or Minimum
- Unit: target dosage (%; mg/kg; mg/L)
- Target value: allows you to specify the value to be monitored.
Agri-food examples: quantity with yield, quantity as prepared, maximum quantity of the ingredient.
Cosmetic examples: synthetic, natural.
- Ingredient level: indicates the specific level to be monitored. Cosmetic example: direct (1) or impurities (2).
- GMO: indicates whether the use of ingredients containing GMOs is autorized or not.
- Ionization forbidden: indicates whether the use of ionized ingredients is autorized or not.
- Geographical origins: allows the use of ingredients from certain countries to be forbidden.
- Required geographical origins: allows you to define a mandatory origin for ingredients. In this case, you should not specify a maximum dosage and should use the ‘Forbidden’ requirement level.
- Biology origins: allows the use of ingredients from certain crops to be forbidden.
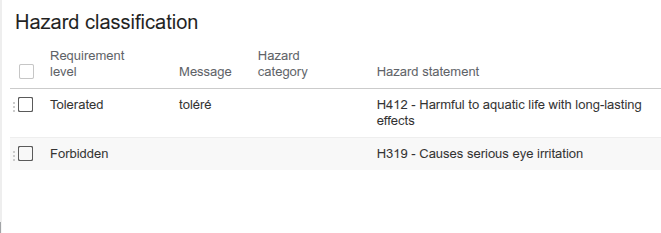
Hazard classification
This feature generates an alert if certain hazard statements are present in the recipe.
In this example:
- the phrase "H412 - Harmful to aquatic life with long-lasting effects" is tolerated in the recipe.
- the phrase "H319 - Causes serious eye irritation" is forbidden. ;
Note: Unlike other lists, there is no ‘Compliances’ column; the alert is displayed directly in the completion wheel.
Score
This feature allows you to define criteria with categories and then generate scores on entities associated with the specification by inheritance.
Criterion: select a criterion configured in Administration
Parent: allows you to create categories to generate a score and sub-scores
Here is the type of score you can generate on your entity:

For creating criteria, please refer to the documentation on project lists. Project lists
Note: Parent criteria can only be defined in the specifications. If criteria are added directly to the entity model, it will not be possible to prioritize them.
Labeling rules
In cases where several products share the same rules, and in order to avoid creating specific rules for each one, it is possible to define common labeling rules at the specification level that apply to the products associated with that specification. Thus, when creating a new product linked to this specification, these rules will automatically be taken into account when generating the labeling.
To create labeling rules, refer to the documentation. Labeling rules
Non compatible products
WARNING : Please consult your beCPG project manager before implementing this feature to ensure that there are no contraindications for your system.
Ensure that the ‘Non compatible products’ list is displayed before you begin. This feature allows you to quickly check whether certain RM, SFP, or FP comply with your specifications, based on prohibited characteristics.
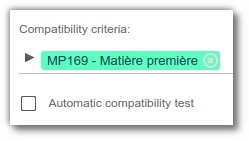
1. Entity selection
Go to the ‘Properties’ tab.
Fill in the ‘Compatibility criteria’ field: Select the types of entities to be checked: Raw material, Finished product, etc.
2. Application of rules
- Create your criteria in the specification
- Click ‘Formulate’ in the Properties tab.
- Entities that do not comply with the rule will automatically appear in ‘Non compatible Products’.
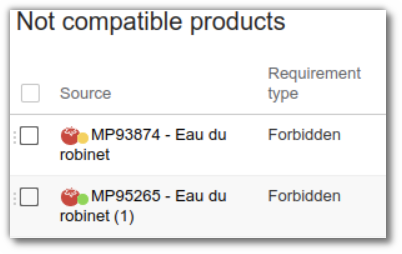
The tab takes into account all prohibitions, including geographical and biological ones.
Note :
- Only prohibitions with a maximum value (%) are taken into account.
- The ‘Non compatible Products’ list only takes into account prohibitions for which a maximum value (%) has been defined in the case of an ingredient prohibition. To completely forbidden a product, enter 0%.
- To temporarily disable a forbidden, leave the value blank.
- Add a clear message in the ‘Details of requirements’ column for each rule.
- Create one rule per forbidden so that each message remains specific.
Regulatory conformities of export products
To use the regulatory conformitie feature, you must create a specification for each regulatory country (e.g. USA, EU) and for each regulatory use (e.g. Food additive). This information must then be entered in the specification properties. The specification must then be linked to the product in order to apply.
Conformities list for finished products
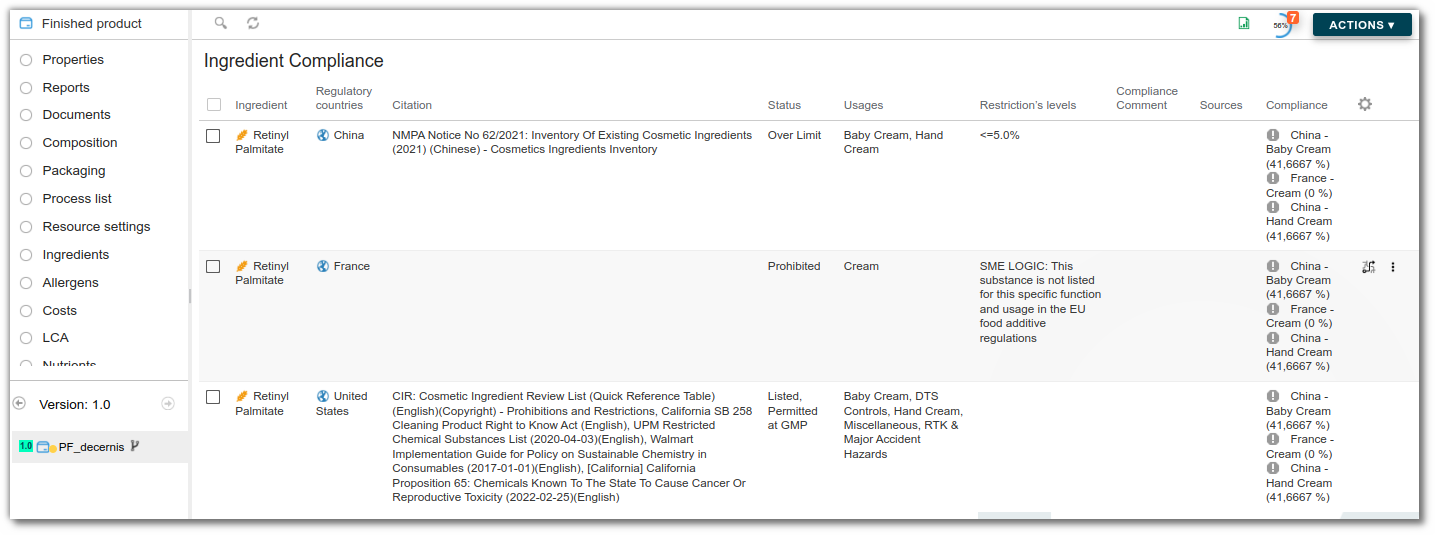
An entity's ‘Conformities’ list allows you to check the exportability of products. When a specification stipulates that an ingredient or allergen is prohibited in a given country, the word ‘prohibited’ appears on the corresponding line.
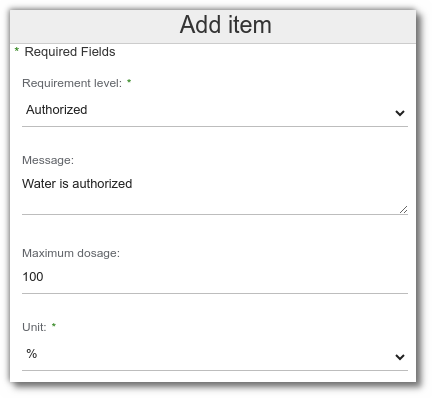
To view the compliance results by country and regulatory use, follow these steps:
- In the ‘Conformities’ list for your finished product, select the country and regulatory use on the same line.
- On this line, the following is displayed after formulation:
- the regulatory result,
- the limiting elements,
- the maximum authorized dosage for which the recipe may be used, calculated according to the following formula :
(% of dosage entered in the specification / % of ingredient in recipe) * 100
Example :
The ingredient oleic acid has a restriction of 2% in the specification. It is present at 44% in the recipe.
(2%/44%)×100=4,5455
Therefore, the maximum dosage for use in the recipe is 4.55%.
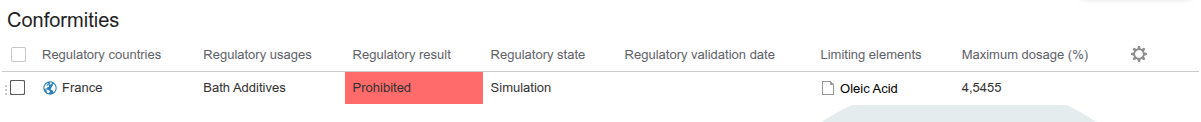
Regulatory result based on the level of requirement
The regulatory result varies depending on the level of requirement defined:
- Forbidden : The compliance result displays ‘Prohibited’ if the recipe contains an ingredient that is prohibited in the specification.
- Tolerated : If the compliance result displays ‘Permitted’, the ingredient is accepted despite the restriction.
- Authorized : The compliance result displays ‘Prohibited’ for any ingredient not present in the specification.
- Information : If the compliance result displays ‘Permitted’, this is for information purposes only.
Decernis
This list will always work with beCPG specification and can also be combined with data from Decernis depending on the "Regulatory Mode" entered in the entity properties:
- Only visible in external database: Decernis results are only taken into account in Decernis
- Only visible in beCPG: Decernis results are only taken into account in beCPG
- Visible in both: Decernis results are taken into account in Decernis and on beCPG
- Disable external database: Decernis results are not taken into account on either beCPG or Decernis
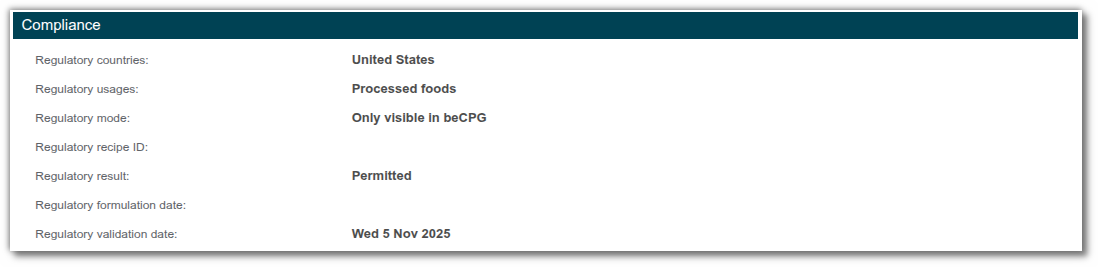
Ingredient Regulatory List
The ‘Ingredient Regulatory’ list on finished products only works when interfaced with Decernis. It provides more detailed information on each forbidden ingredient and explains the reason for its prohibition.